Astronomy and Archaeology are very similar professions in a way that the subject of study in both cases are neither in the vicinity of the researcher in space nor happening in the present time. The only way that people in these professions can work is by making a story out of the little bits of information that reaches them and then verify those stories on the basis of scientific and logical correctness. Life is easy as a chemist when you can mix two chemicals in your lab and watch the reaction unfold or as a biologist when you can dissect an insect to study its anatomy. Nonetheless, this very art of storytelling is what makes Astronomy so fruitful and satisfying. When you are able to determine the chemical composition of a star thousands of light years away or calculate the velocity of a galaxy. Except in the study of nearby planets and meteorites, it is not feasible to receive material information from astronomical sources. We have sent human missions to the Moon to take soil samples. Sometimes we receive a meteor from the outer worlds that carry valuable elemental and physical information. We have sent probes further to transmit information back and some to return with samples from celestial bodies. Perhaps, in the next few decades we will send a probe to the nearest star. What about the countably infinite stars and galaxies that stretches across the night sky? Presently, the most common way by which information reaches us from distant astronomical sources is in the form of Light or Electromagnetic Radiation.
We are constantly showered with
electromagnetic waves and cosmic rays from the sky. They are the most
fundamental way by which we perceive information from the Universe; constituting
of periodic disturbances in the electric and magnetic field, the existence of
these waves was first properly suggested by James K. Maxwell through his famous
Maxwell’s Equations. Today, we know a lot about the nature of light. From the
classical wave nature to the quantum particle nature, light comes in all sorts
of colors and energies. The 19th and 20th century was a
wonderful time for Astronomy and Physics as parallel breakthroughs in these
fields were taking place almost simultaneously. These groundbreaking
discoveries were facilitated by each other and contributed to a lot of the
Modern Physics we know today.
The most important moment in
Astronomy was when physicists decided to take a prism and point it at the light
coming from the Sun. A prism is a special optical device made of glass, which
splits white light into its constituent colors – Violet, Indigo, Blue, Green,
Yellow, Orange, and Red. Each of these colors of light differ in terms of their
wavelengths and frequencies which is the distance between two consecutive peaks
or troughs of the waves and the number of oscillations in one second
respectively. The light of red color has the greatest wavelength and least
frequency while violet color has the least wavelength and highest frequency.
Although their frequencies and wavelength differ, all of the colors travel at
the same speed of 3 lakh kilometers per second. In 20th century, an
important discovery in Quantum Physics proved that the energy of a photon is
directly proportional to its wavelength and further that light isn’t emitted or
absorbed continuously but in discrete chunks of energy. This meant that blue
light has more energy as compared to say red light. However, the spectrum of
electromagnetic radiations is not just limited between Violet and Red. It
extends further on either ends into the more energetic Ultraviolet, X-Rays and
Gamma Rays and the less energetic Infrared, Microwaves and Radio waves.
 |
| Fig 1 : Dispersion of White Light by a Prism |
The study of hot gases was pivotal in
the development of early Astronomy. In the simplest language possible, if one
takes any element in a gaseous state(hydrogen for e.g.) at low energies and subjects it to a
polychromatic (multiple wavelengths) light source. The spectrum of such light
source obtained after passing it through a prism has a characteristic property.
This spectrum when viewed on a screen can be observed to have vertical dark
lines at specific positions(Fig 2). The normal spectrum of white light indicates lights of
different frequencies (color) from left to right. Each position on that
spectrum corresponds to a light of a specific frequency. Although the spectrum
is made of continuous bands, we can slice its portion into vertical lines, with
each line representing a fixed frequency. Therefore, in the second spectrum
obtained after passing the light through hydrogen gas and then the prism, the
observed dark lines indicate absence of light of that frequency. Since, those
dark lines are observed only when hydrogen gas is present in the path of light,
it can easily be concluded that gaseous hydrogen is absorbing some of the light
of specific frequencies only. The quantum explanation for this is that each
atom of hydrogen in that gas is composed of two parts – the central, positively
charged nucleus and the negatively charged electrons orbiting it. These
electrons orbit the nucleus at fixed energy levels at a certain distance from
the nucleus. Most of the times, an electron can absorb energy either in the
form of light or heat and jump up to the higher energy level. However, the
electron has to absorb that energy which is perfectly equal to the energy
difference between the two levels. In case of light, this would mean it can
absorb only those photons whose frequency corresponds to that energy
difference.
It is as if, there are molds of
specific shapes which take in a continuous fluid to form shapes like triangle,
circle, etc. whilst leaving the same shaped gaps in the fluid. This spectrum of
light after passing it through a cold fluid of specific elements is known as
the – “Absorption spectrum” of that element. The absorption spectrum of each
element is unique and is characterized by the position of dark lines in that
spectrum. Thus, by observing the signature absorption spectra of elements one
could determine the name of that element. What if we repeat the same experiment
but this time, instead of a light source we heat up the hydrogen gas and energize
it. As you might have guessed, this will have the opposite effect as compared
to the absorption spectra. When the gas is energized, electrons in the atoms of
that gas jump down from higher energy levels to lower energy levels. In order
to conserve the energy, the atom emits a photon (light particle) having the
same energy as the energy difference between the two levels. If you now observe
the spectrum of light coming from such an energized gas, you would observe
vertical lines of fixed frequencies against a completely black background. The
lines are of a single color (monochromatic), indicating that the hydrogen gas
only emitted light of specific frequencies. Furthermore, as one might expect,
these color lines are exactly at the same position in the spectrum where the
dark lines are observed in the absorption spectrum. This spectrum obtained from
the light emitted by a hot or energized element is known as the “Emission
Spectrum” of that element. If you overlap the emission spectrum of an element
over its absorption spectrum, then the position of dark and colored lines would
perfectly coincide and you would retrieve the ordinary spectrum of white light.
The final take away from this is that every element can absorb or emit light at
specific frequencies, this result in absorption or emission spectra of
different elements which is unique for every element.
| Fig 2 : Ordinary spectrum of white light vs Emission and Absorption Spectrum of an element. |
Returning to the story of stars: In
the 19th century, Joseph Fraunhofer who was a German physicist and
an optician analyzed the spectrum of light coming from our Sun and many other
stars. He mounted a prism to the eyepiece of his telescope and pointed the
telescope at those stars. To his surprise, Fraunhofer noticed vertical, dark
lines in those spectra at specific positions. He precisely labeled the set of
these dark lines according to their positions, which became known as
“Fraunhofer lines”. Many years later, the similarity of Fraunhofer lines to the
absorption spectra of certain gaseous elements was discovered. The implication
was clear, there was a presence of these gaseous elements in those stars which
were absorbing specific frequencies of light emitted by those stars, giving
rise to the “absorption spectra”. In case of our Sun, it produces light as an
almost continuous spectrum, but as the light passes through the various layers
of atmosphere and photosphere of the Sun, it gets absorbed at various
frequencies giving rise to the Solar spectrum as shown below :
 |
| Fig 3 : Fraunhofer Lines in the Solar Spectrum |
It is a common observation that whenever a vehicle or a train engine is approaching at some velocity, then the pitch of its sound rises progressively until it crosses you and then recedes as the sound source moves away. This behavior of sound is a consequence of its wave nature. Sound propagates through the medium of air in the form of longitudinal waves which are back and forth variations in the air pressure. As the source starts moving in one direction, if it emits one cycle of the sound wave at some instant, then by the time it emits the second cycle, the source would have moved closer to the first cycle (pressure compression) and so the second cycle of wave is emitted in a shorter time interval than it would have if the source were at rest. Owing to this, the moving source emits more cycles of sound waves in a shorter timespan thus making it sound at a higher frequency or pitch for a ground based observer. This effect is known as the “Doppler Effect”.
| Fig 4 : Doppler Effect of a moving sound source |
Since, light also is an electromagnetic wave, this effect is prevalent in the propagation of light waves as well. If any object is moving towards an observer on Earth with sufficiently high velocities then the high pitch equivalent of light coming from it would be a shift of the light towards the blue end of spectrum because blue color corresponds to a higher light frequency. Similarly, for an object receding away from us, the light coming from it would be shifted towards the red end. Such light is called as “blue shifted” or “red shifted” and was used by the famous astronomer Edwin Hubble in the 1920s to discover that galaxies are moving away from us and so the Universe is expanding and non-static, which was contrary to what Einstein and many other physicists believed. This shift of light is observed in the spectrum of any astronomical object. For example, let’s say you analyze the spectrum of a star A and note the positions of the Fraunhofer lines corresponding to some elements. In order to do this, you compare the spectrum of that star to a reference spectrum of elements obtained in the laboratory. The positions of the dark lines in emission spectra give you an idea of what elements are present. However, you notice something peculiar in the spectrum of star A. The dark lines which you observed in the reference spectrum are not at the same position in the emission spectrum of star A but are shifted by a fixed amount towards the blue end of the emission spectrum. The spectrum of that star is called to be “blue shifted”. It can then be deduced that star A is moving towards us, this causes the light emitted by it to be increased in frequency. Similarly, the spectrum of stars moving away from us becomes “red shifted”.
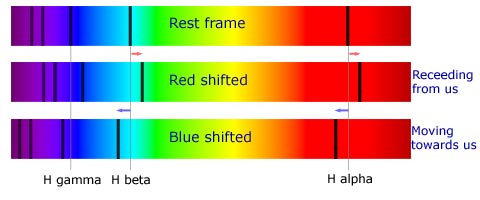 |
| Fig 5 : Red Shifted and Blue Shifted spectra compared to a spectrum in rest frame |
The Doppler Effect in stellar spectra
soon became an important tool to calculate velocities of stars and even galaxies
on the cosmological scale. These calculations yielded precise velocities of
stars in binary and more complicated star systems. The radial velocity method
was used to detect and measure the wobbles produced in a star because of the
gravitational tugging of a potential exoplanet. The velocities allowed estimating
masses of star in star systems by simple mechanical calculations.
In the 20th century,
developments in Quantum Mechanics and Thermodynamics found its applications in
the field of Astronomy and Astrophysics. In Thermodynamics, one of the main
concerns is the ways in which heat energy can be absorbed or transmitted by a
body. You must have noticed whenever you bring your hand close to a heated pan
or a piece of metal; you could feel the heat without touching the pan. This is
because when the pan gets hot, its molecules and atoms start to jiggle around
randomly and emit radiations which are nothing but electromagnetic radiations
mostly of the infrared and microwave regions. These radiations are then
incident on the atoms of your skin, which absorb them and get excited in turn,
producing heat. The amount of heat radiation that a body can absorb depends
mostly on its material and shape. In thermodynamics, one imagines an ideal body
which could absorb all of radiation incident on it. Such a body is called as
“Blackbody”. Although, no object could be regarded completely as a “blackbody”,
there are some cases in which an object could be approximated pretty closely as
a blackbody. Experiments were conducted with such blackbodies to study their
nature and as a result various laws were discovered. The most important of them
was the – Wien’s Displacement Law. The law states that the wavelength of the
radiation of maximum intensity emitted by a body is inversely proportional to
its temperature. A body generally emits electromagnetic radiations in all
wavelengths at different intensities. Wien’s law relates the wavelength of this
radiation to the temperature of the body. This relation is such that the
wavelength of emission is inversely proportional to the body temperature, or
the frequency of emitted radiation is directly proportional to the temperature.
Consequently, a hot body will emit radiation of higher frequency than that
emitted by a cooler one. So, bodies at higher temperatures will glow with a
bluish hue and those at cooler temperatures will appear reddish. Alternatively,
this result can also be explained by the energy – frequency relation given by
Planck, which we saw earlier. Bodies at higher temperatures have more heat
energy and hence will glow at higher frequencies and vice versa.
In spectroscopy, this thermodynamic
relation was used to estimate the temperature of stars and celestial bodies
which emit radiation. The relative brightness of the different colors in a
stellar spectrum is compared, and the color with greatest brightness
(intensity) is used to calculate the temperature of a star. If the maximum
intensity is more towards the bluer side of spectrum, then the star itself
appears bluish and has a very high temperature. Similarly, if the maximum
intensity is towards the redder side of spectrum then the star has a low
temperature. Therefore, a common observation in Astronomy was that red stars
are cooler than blue stars. On the basis of their color and respective
temperatures, the stars can be classified into different types. This is known
as the Harvard Spectral Classification and all the stars are roughly divided
into 7 broad categories from hottest to coolest as : O, B, A, F, G, K, M. The
stars from O to F are blue or bluish-white in appearance and have a hot
temperature of 6000 to above 25,000 kelvins.
The stars from G to M are yellowish white to red in appearance and have
a relatively cooler temperature of 3000 to 5000 kelvins. Our Sun belongs to the
G type and glows in white color with an intermediate temperature. It appears
yellow – orange from Earth due to atmospheric scattering effects.
.png) |
| Fig 6: Spectral curve peak of different stars according to their temperature |
What appeared to be a simple result
of placing a glass object in the path of light rays was employed so extensively
in the domains of Astronomy. A simple glass prism enabled us to calculate the
chemical and physical properties of stars at vast distances. Perhaps, one could
take this as a prime example for the tremendous potential of seemingly trivial
discoveries in Physics. The current state of Modern Physics is often questioned
for its benefaction to human society. Nevertheless, I believe that with time
and progress, the importance of gravitational waves, particle accelerators, and
black holes will soon be realized similar to the importance of the rainbow obtained from a prism.
-
Thank you.